Have you ever wondered what makes a battery work? Most batteries contain acid. This acid plays a key role in creating energy. It’s almost like magic! But how does it really work?
Think about your favorite toys that need batteries. Without the acid inside, they just won’t run. Many people don’t realize that the right acid helps keep your devices powered up. Isn’t it surprising that such a simple ingredient can make a big difference?

In this article, we will dive into the world of acid for batteries. We’ll explore how it works and why it’s so important. Get ready to discover the secrets that keep your gadgets alive! Stay tuned; you won’t want to miss this!
The Best Acid For Batteries: Choosing The Right Type
Many people don’t realize that acid is crucial for battery function. This liquid helps create a chemical reaction that produces electricity. Without it, most batteries wouldn’t work. Did you know that car batteries use sulfuric acid? It’s true! Acid can be dangerous, so always handle batteries carefully. Understanding how acid works can help you understand why your devices run out of power and when to recharge them. Batteries keep our world powered, so knowing how to care for them is important!
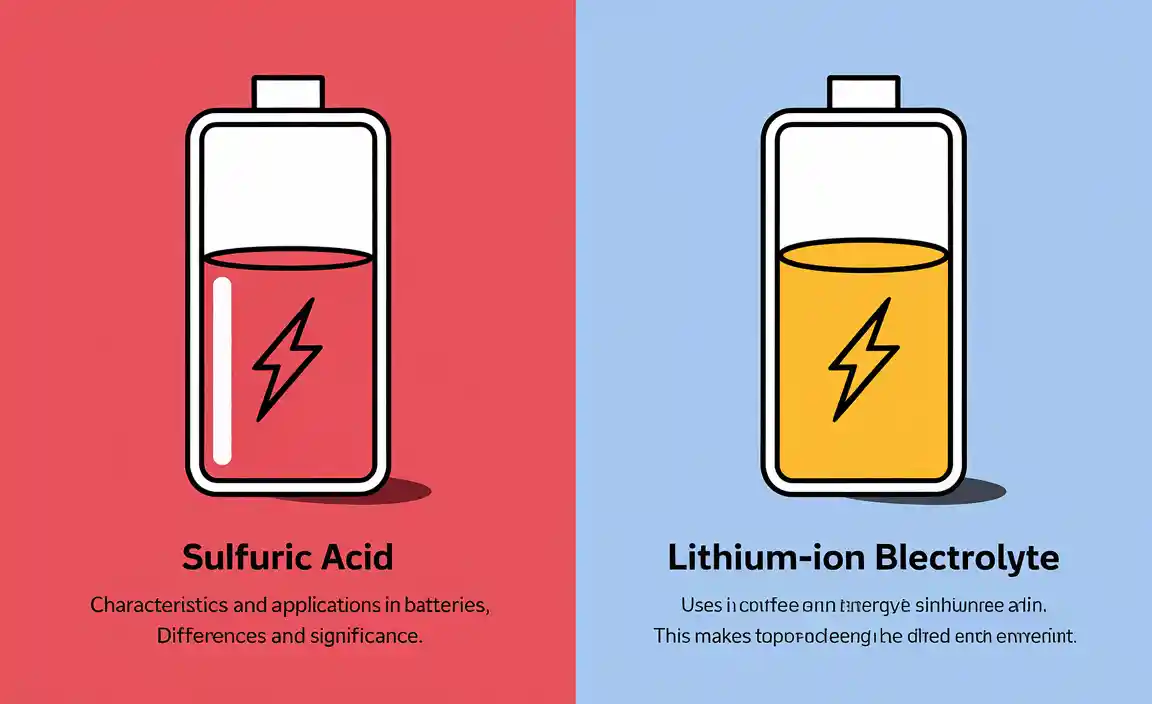
Types of Battery Acid
Sulfuric acid: Characteristics and applications in leadacid batteries. Lithiumion battery electrolyte: Differences and significance.
Batteries use different types of acid. One common type is sulfuric acid. It is mainly found in lead-acid batteries. This acid helps store energy effectively. Another type is in lithium-ion batteries. It uses a different electrolyte. This makes lithium-ion batteries lightweight and efficient. Here’s a quick comparison:
- Sulfuric Acid: Used in lead-acid batteries; heavy but reliable.
- Lithium-ion Electrolyte: Lightweight; faster charging and longer life.
Understanding these differences helps choose the right battery for your needs!
What is sulfuric acid used for in batteries?
Sulfuric acid is mainly used as the electrolyte in lead-acid batteries. It helps in the chemical reactions that produce electricity.
How Battery Acid Functions
Electrochemical reactions: The role of acid in energy storage. Conductivity and stability: Ensuring efficient battery operation.
The magic of battery acid lies in its chemistry! It helps store energy through electrochemical reactions. This means when you charge your battery, the acid plays a special role in moving electrons around, like a little dance party for atoms. When it comes to conductivity and stability, the acid ensures electricity flows smoothly, keeping your devices powered up. Without it, your battery might as well be a rock—pretty useless!
| Function | Importance |
|---|---|
| Electrochemical Reactions | Stores energy effectively |
| Conductivity | Ensures efficient operation |
| Stability | Keeps devices running smoothly |
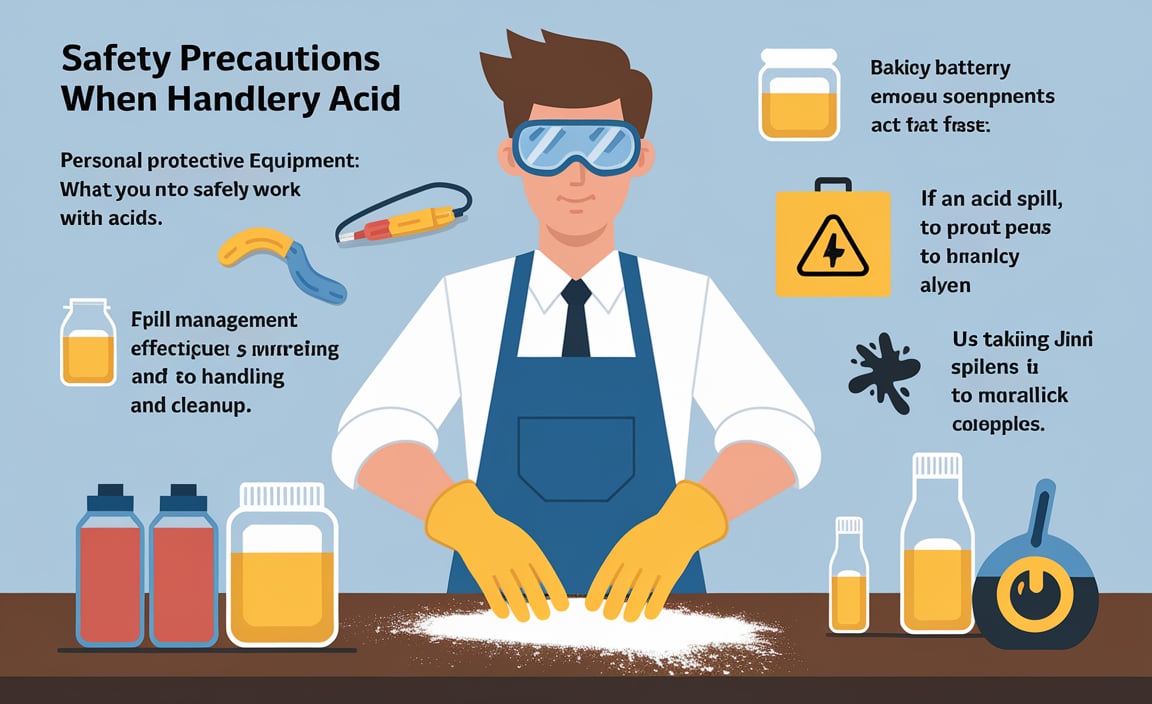
Safety Precautions When Handling Battery Acid
Personal protective equipment (PPE): What you need to safely work with acids. Spill management: Effective techniques for acid handling and cleanup.
Handling battery acid can be tricky, but safety should always come first. Wear personal protective equipment (PPE) like gloves and goggles to protect your skin and eyes. You don’t want to end up like a crazy scientist in a lab coat, right? If an acid spill happens, act fast! Use baking soda to neutralize it, then clean up right away. Remember, being safe is key. Don’t let battery acid give you a shocking surprise!
| Safety Gear | Purpose |
|---|---|
| Gloves | Protect hands from acid |
| Goggles | Shield eyes from splashes |
| Apron | Prevent acid from contacting clothes |
Maintenance of Acid in Batteries
Importance of checking acid levels: Ensuring optimal performance. Best practices for topping up battery acid: Dos and don’ts.
Keeping an eye on battery acid levels is key. It helps batteries work their best. Low acid can cause damage and shorten battery life. Regular checks ensure optimal performance. Not sure how to top up the acid? Here are some tips:
- Always wear gloves and goggles for safety.
- Use distilled water to avoid harmful minerals.
- Don’t overfill the battery. Leave some space.
- Never use tap water; it can harm the battery.
Following these simple rules will help your batteries last longer and work better.
Why is checking battery acid important?
Checking battery acid is important because it keeps the battery running properly and extends its life.
Best time to check battery acid:
- When the battery is low on charge.
- Every few months.
Environmental Impact of Battery Acid
Disposal regulations: Guidelines for responsible acid disposal. Recycling options: How to recycle used batteries and their acids.
Taking care of battery acid is a big deal! It’s not just a slippery subject but also a sticky one. To start, there are strict rules for disposal. You can’t just toss it in the trash; that would be a no-no. Be sure to follow the local guidelines to avoid making the Earth frown.
Recycling is a superhero move! Used batteries can be recycled through special programs. This not only helps the environment but also can keep some bad stuff out of your backyard. Common places to recycle are local dumps or electronics stores. So, instead of letting old batteries hang out in a closet, give them a new life!
| Disposal Method | Details |
|---|---|
| Local Waste Management | Check local rules on how to dispose of battery acid safely. |
| Drop-off Centers | Find a center that accepts used batteries for safe recycling. |
Innovations in Acid Technology for Batteries
Emerging battery technologies: Alternatives to traditional acid batteries. Future trends: Research and development in acidbased battery systems.
New battery technologies are changing how we think about energy. Research is focusing on alternatives to traditional acid batteries. For example, companies are exploring lithium, solid-state, and flow batteries. These options can offer longer life and faster charging. Experts believe these innovations may lead the way to better batteries in the future.
Next, researchers are diving deeper into acid-based systems. They aim to improve performance and safety. They also look at how to recycle these batteries. This research is key to a cleaner, more sustainable future.
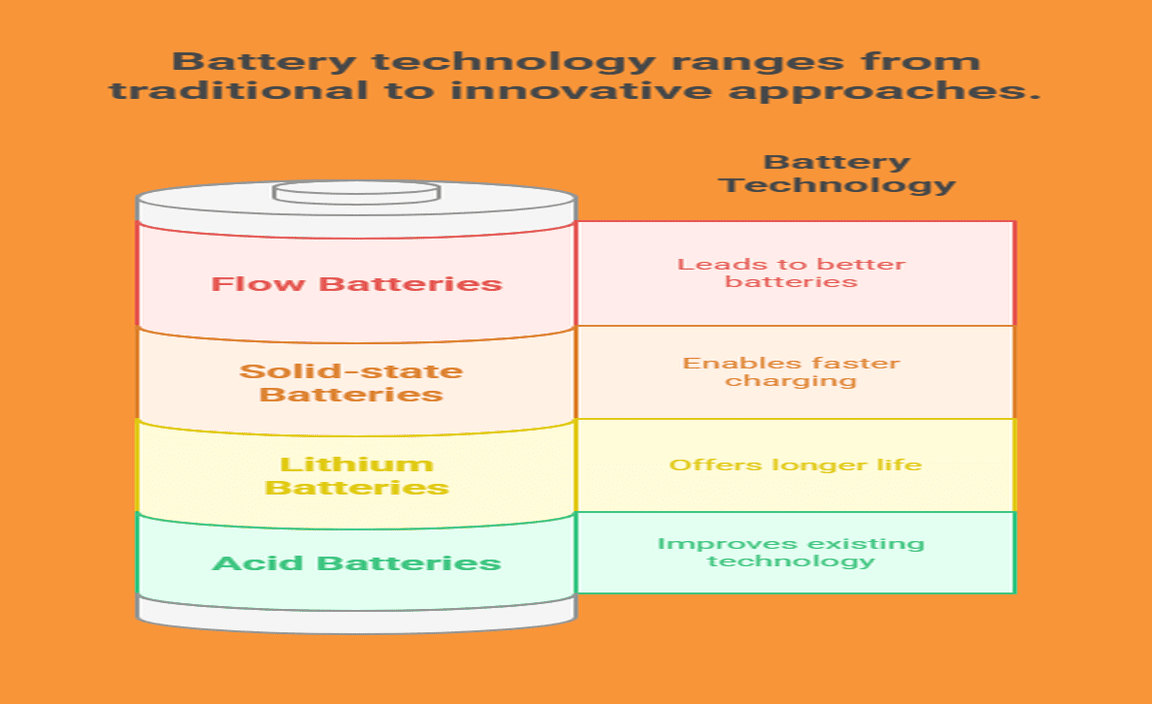
What are the alternatives to traditional acid batteries?
Some alternatives include lithium-ion, solid-state, and flow batteries. These newer types offer better efficiency and can last longer than acid batteries.
Future Trends in Battery Technology
- Improved safety features
- Faster charging times
- Better recycling processes
Common Problems Related to Battery Acid
Acid stratification: Causes and effects on battery performance. Corrosion and damage: Identifying and addressing battery acid leaks.
Battery acid can be a tricky business! One issue is acid stratification. This happens when acid settles into layers, making your battery lazy and less powerful. It’s like a battery nap when it should be running a marathon! Another problem is the sneaky corrosion from acid leaks. If you see white or green stuff around your battery, that’s a sign. It’s crucial to clean it up to keep your battery healthy. Here’s a quick summary of these common culprits:
| Problem | Causes | Effects |
|---|---|---|
| Acid Stratification | Improper charging | Reduced performance |
| Corrosion | Acid leaks | Battery damage |
Conclusion
In conclusion, acids are vital for battery operation. They help store and release energy efficiently. Understanding how acids work can improve your battery use. You can keep batteries longer by maintaining them properly. If you’re curious, explore more about battery types and care. This knowledge will help you make smart choices for your devices!
FAQs
Sure! Here Are Five Related Questions On The Topic Of Acid For Batteries:
Sure! Battery acid helps batteries store and give out power. It usually comes from sulfuric acid, which is a strong liquid. You should never touch battery acid because it can be very harmful. Always ask an adult for help when dealing with batteries. They know how to handle them safely!
Sure! Please ask your question, and I’ll provide a short, simple answer.
What Types Of Acid Are Commonly Used In Lead-Acid Batteries, And How Do They Function Within The Battery System?
Lead-acid batteries use sulfuric acid as the main type of acid. This acid helps the battery store and release energy. When you charge the battery, the acid reacts with lead plates inside. This process creates electricity, which powers things like cars and UPS systems. When the battery discharges, it gives energy back to help things work.
How Does The Concentration Of Sulfuric Acid In A Lead-Acid Battery Affect Its Performance And Lifespan?
The concentration of sulfuric acid in a lead-acid battery is very important. If the acid is strong enough, the battery can store more energy and work better. But if it’s too weak, the battery won’t last as long and might even get damaged. Keeping the right amount of acid helps the battery perform well and live longer. So, we need to check and keep the acid concentration just right!
What Safety Precautions Should Be Taken When Handling Battery Acid During Maintenance Or Disposal Procedures?
When you handle battery acid, wear safety gloves and goggles. This keeps your skin and eyes safe. Always work in a well-ventilated area, so you breathe fresh air. If you spill it, clean it up right away. Finally, be careful not to touch your face while working with it.
How Do Acid Levels In Batteries Influence The Charging Process And The Overall Energy Efficiency Of The Battery?
Acid levels in batteries are very important. When there is enough acid, batteries charge well and hold a lot of energy. If the acid is too low, the battery won’t charge properly and can waste energy. This means keeping the right acid levels helps the battery work better and last longer.
What Are The Environmental Impacts Of Improper Disposal Of Acid From Batteries, And What Are The Best Practices For Recycling Battery Acid?
Improperly throwing away battery acid can hurt our land, water, and animals. The acid can leak into the ground and poison plants and creatures. This makes it dangerous for people, too. To recycle battery acid safely, we should take old batteries to special recycling centers. These places know how to handle the acid without harming the environment.
Resource:
-
Battery Safety Basics: https://www.nfpa.org/Public-Education/Staying-safe/Safety-equipment/Battery-safety
-
Proper Disposal of Batteries: https://www.epa.gov/recycle/used-household-batteries
-
How Lead-Acid Batteries Work: https://www.britannica.com/technology/lead-acid-battery
-
Emerging Battery Technologies: https://www.energy.gov/eere/vehicles/articles/energy-department-announces-major-investments-advanced-batteries